Understanding Quantum Mechanics and Its Implications for Reality
Introduction to Quantum Mechanics
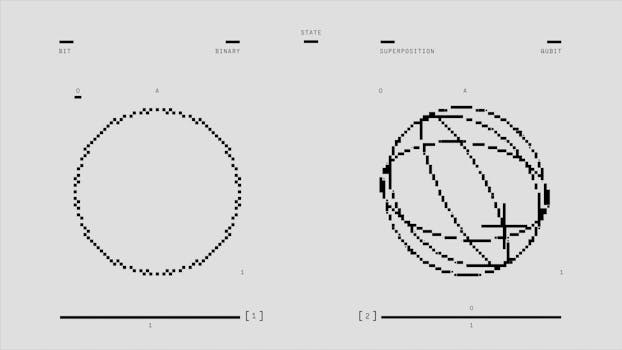
Quantum mechanics is a fundamental theory in physics that describes the behavior of matter and energy at the smallest scales. At these scales, the classical laws of physics no longer apply, and strange, seemingly random phenomena start to occur. Quantum mechanics was developed in the early 20th century by scientists such as Max Planck, Albert Einstein, and Niels Bohr, and has since been extensively tested and confirmed through numerous experiments.
Quantum mechanics is based on the principle of wave-particle duality, which states that particles, such as electrons, can exhibit both wave-like and particle-like behavior depending on how they are observed. This principle is illustrated by the famous double-slit experiment, in which electrons passing through two slits create an interference pattern on a screen, indicating that they are behaving like waves.
Key Principles of Quantum Mechanics

There are several key principles that underlie quantum mechanics. These include:
- Superposition: The ability of a quantum system to exist in multiple states simultaneously.
- Entanglement: The phenomenon where two or more particles become connected in such a way that the state of one particle is dependent on the state of the other, even when they are separated by large distances.
- Uncertainty principle: The principle that it is impossible to know certain properties of a quantum system, such as its position and momentum, simultaneously with infinite precision.
These principles have been extensively tested and confirmed through numerous experiments, and have led to the development of many important technologies, including transistors, lasers, and computer chips.
Implications of Quantum Mechanics for Reality

Quantum mechanics has significant implications for our understanding of reality. One of the most important implications is that reality is fundamentally probabilistic, rather than deterministic. This means that, at the quantum level, the behavior of particles is inherently random and unpredictable, and can only be described using probabilities.
Another implication of quantum mechanics is that the act of observation itself can influence the behavior of quantum systems. This is known as the observer effect, and has been demonstrated in numerous experiments. For example, in the double-slit experiment, the act of observing the electrons as they pass through the slits can cause them to behave like particles, rather than waves.
Conclusion
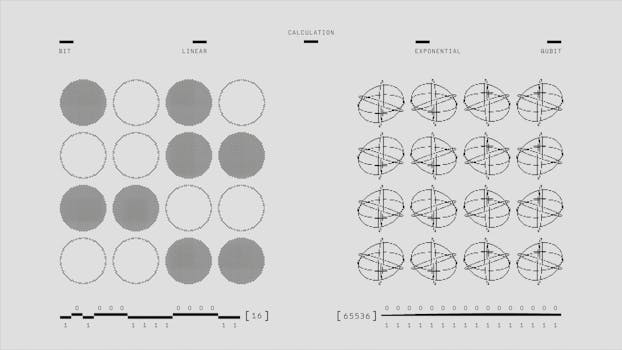
In conclusion, quantum mechanics is a fundamental theory that describes the behavior of matter and energy at the smallest scales. Its principles, such as superposition, entanglement, and the uncertainty principle, have been extensively tested and confirmed, and have led to the development of many important technologies. The implications of quantum mechanics for reality are significant, and suggest that reality is fundamentally probabilistic, and that the act of observation itself can influence the behavior of quantum systems.